How are we different from others
To ensure product quality, we proceed with design verification, production testing, software verification, Image Quality (IQ) testing, and regulation certification. We also implement validation on production equipment.
Quality Management System
We have our own quality control system and devote significant attention to quality control for the designing, R&D, manufacturing, and testing of our products. Our quality management team is actively involved in setting quality policies and managing our internal and external quality performance. We have established a strict quality control system in accordance with FDA or comparable regulations, ISO 13485 standards, and EU regulations on the quality management system of medical devices.

Image Quality Testing
Altek Medical's parent company, the Altek Group, has been a leader in the digital imaging industry for more than two decades, Altek Medical enhances imaging technologies in medical devices, including image quality testing technologies.
Our core competencies have allowed us to design, commercialize and produce medical devices that are of industry-leading accuracy standard and can provide high-quality images, two attributes that are of significant importance for medical device products, as good image quality will enable diagnostic information to be provided for medical diagnosis and high accuracy in medical devices will ensure safety and certainty in medical treatment.

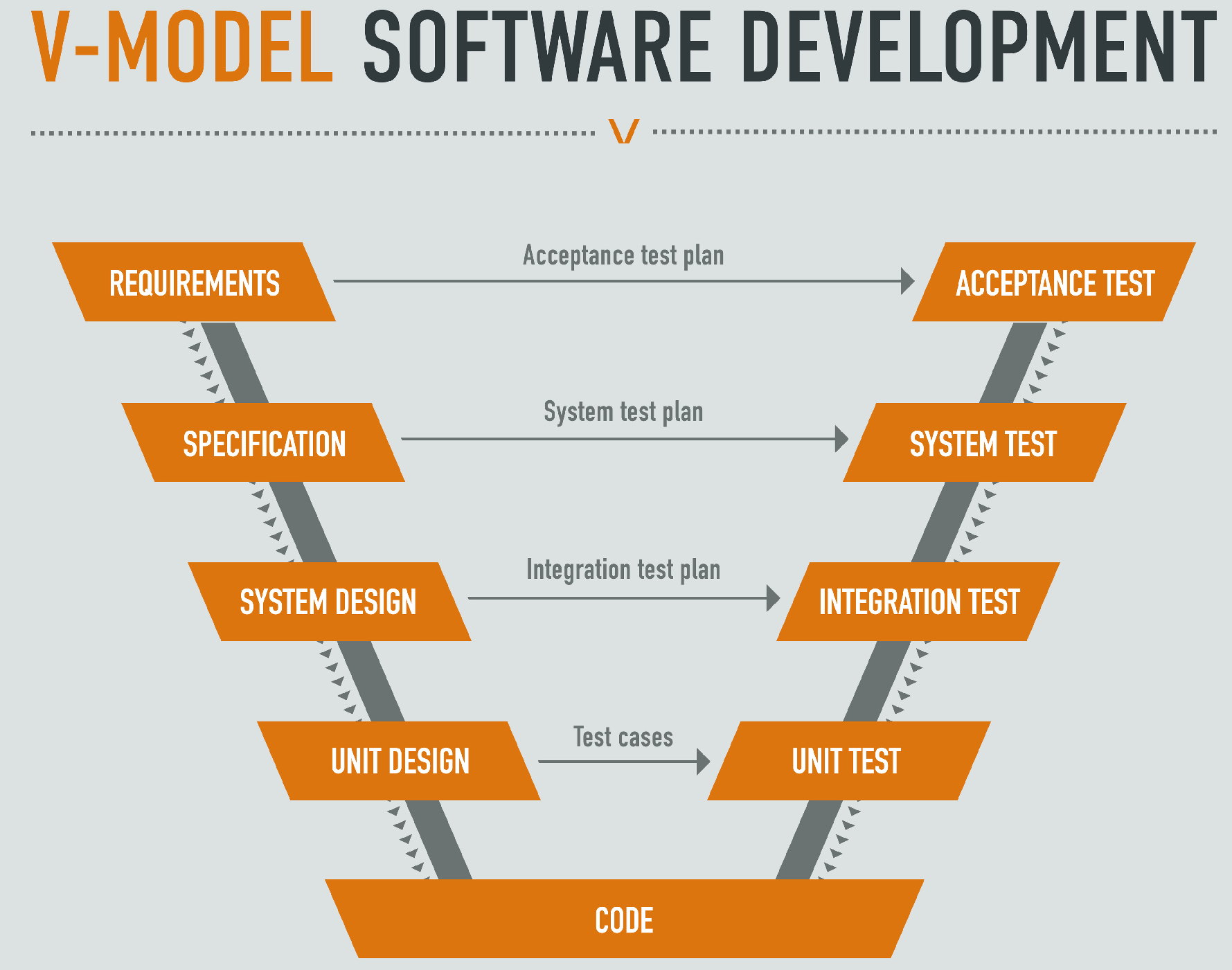
Extremely rigorous software verification
The IEC 62304 defines software that may cause death or serious injury as ultimate Class-C. The Class-C software engineering requires rigorous design, complete documentation, and strict testing. Altek Medical follows the V-Model process to develop and verify the software, our Class-C software expertise has been certificated by international standards, and the product with class-C software was granted with mass production commenced in 2018.